Work function, ionization potential, and electronic density of states measurement
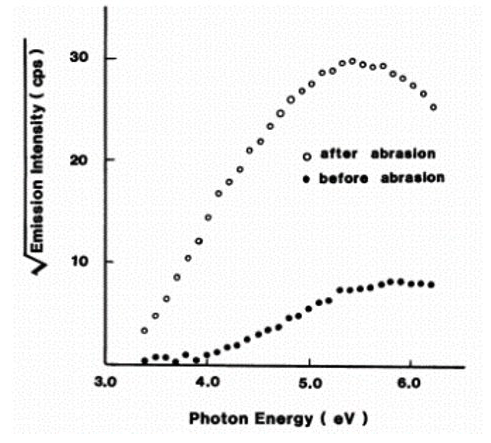
The papers [1] and [2] describe the results of measuring photoelectrons emitted from aluminum. The paper discusses the work function by creating a graph (photoelectron yield spectrum) plotting the photon energy of the irradiated ultraviolet rays against the photoemission and using this to determine the photoemission threshold energy.
Fig. 2: Photoemission behavior from an aluminum plate [1]
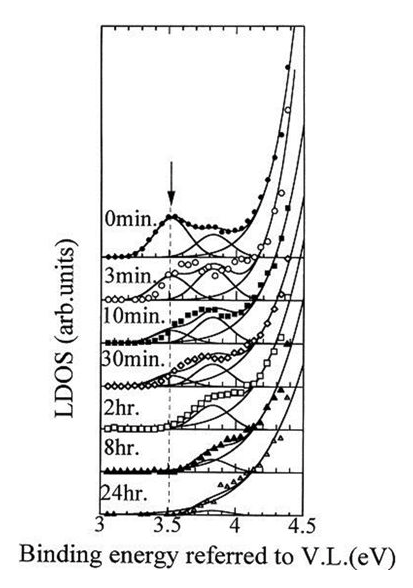
This shows that the work function for a scratched aluminum surface is smaller than that for an aluminum surface left to stand in the atmosphere. These results are measured in greater detail and reported on later [6]. Paper [6] suggests that the work function decreases due to changes to the lattice spacing of the aluminum during the initial oxidation process. In the paper, the authors discuss the changes in the local density of states (LDOS) of the aluminum surface exposed to the air estimated experimentally from the photoelectron yield spectrum compared to the results of molecular orbit calculations.
Fig. 3: Changes in local density of states (LDOS) of an aluminum surface exposed to the air [6]
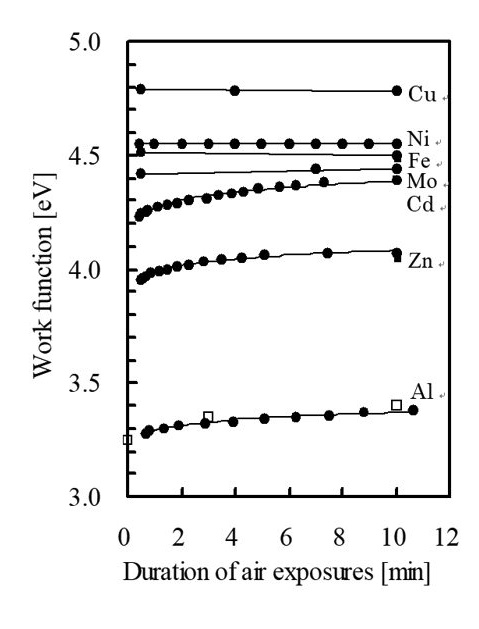
Note that the phenomenon in which the work function of a scratched surface changes can be observed using a Kelvin probe. It has also been reported to occur in zinc and cadmium in addition to aluminum [7].
Fig. 4: Changes in work function of a metal with a scratched surface [7]. The vertical axis represents the work function. The horizontal axis represents the time elapsed after scratching. □ indicates measurements obtained with photoemission yield spectroscopy ● indicates measurements obtained with a Kelvin probe.
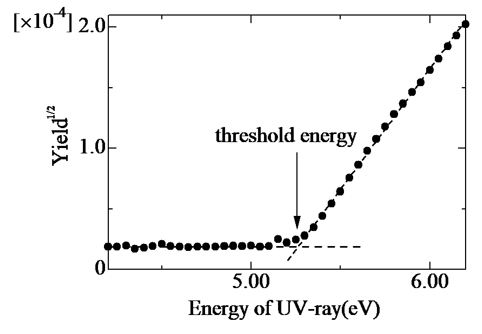
These measurement and analytical techniques have contributed immensely to the development of organic materials. They are widely used in measuring the ionization potential of materials such as organic light-emitting diode materials and Perovskite solar cell materials. In papers [8] and [9], the authors estimate ionization potential and density of states, and compare these against molecular orbit calculations.
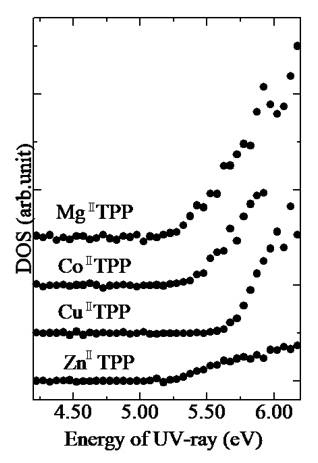
Fig. 5: Relationship between ZnII-TPP irradiated UV energy and the square root of photoelectron yield [8]
Fig. 6: TPP density of states (DOS) obtained from photoelectron spectra [8]
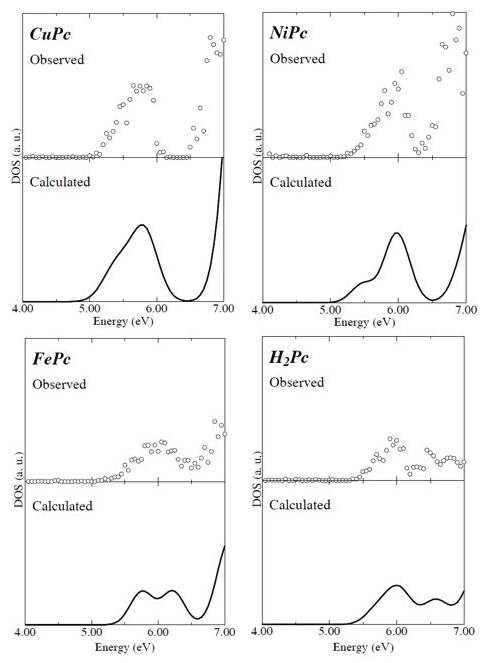
Fig. 7: Comparison of phthalocyanine density of states obtained from photoelectron spectra (upper) against results calculated using DV-Xa method (lower) [9]
[6] M. Uda, Y. Nakagawa, T. Yamamoto, M. Kawasaki, A. Nakamura, T. Saito, and K. Hirose, J. Electron. Spectrosc. and Related Phenom. 88-91, 767 (1998).
https://doi.org/10.1016/S0368-2048(97)00237-5
[7] Y. Nakajima, T. Ishiji, N. Nakano, and M. Uda, “Serial Measurements of the Work Function of Metals Exposed to Air Using a Contact Potential Difference Method”, Journal of the Surface Finishing Society of Japan, 51, 861 (2000).
https://doi.org/10.4139/sfj.51.861
[8] Y. Nakajima, M. Hoshino, D. Yamashita, and M. Uda “Near Edge Structures of Tetraphenylporphyrins Measured by PESA and Calculated with DV-Xα” Adv. Quantum Chem. 42 (2003) 399.
https://doi.org/10.1016/S0065-3276(03)42063-7<
/>>
[9] D. Yamashita, Y. Nakajima, A. Ishizaki, and M. Uda, “Photoelectron spectrometer equipped with open counter for electric structures of organic materials”, J. Surf. Anal. 14,433 (2008);
http://www.sasj.jp/JSA/CONTENTS/vol.14_4/Vol.14%20No.4/Vol.14%20No.4%20433-436.pdf