Gas characteristics and hazards
Describes the characteristics of and hazards associated with main gases. Safety management using gas detectors is important because even gases generally considered safe can pose hazards in certain situations.
Describes the characteristics of and hazards associated with main gases. Safety management using gas detectors is important because even gases generally considered safe can pose hazards in certain situations.
Oxygen is vital to life on our planet. It’s also essential for a wide range of industries that support daily life, including steel manufacturing, chemicals, paper manufacturing, and medicine. However, it also poses certain hazards. Here we’ll present some potentially life-saving facts about oxygen.
Accounting for approximately 21 % of Earth’s atmosphere, oxygen is essential for humans and other creatures.
| Atmospheric gas component | Percentage by volume | Partial pressure |
|---|---|---|
| Oxygen | 20.93 % | 151.1 mmHg |
| Nitrogen | 78.09 % | 593.5 mmHg |
| Carbon dioxide | 0.03 % | 0.2 mmHg |
| Argon and other rare gases | 0.95 % | 7.2 mmHg |
| Total | 100.00 % | 760.0 mmHg |
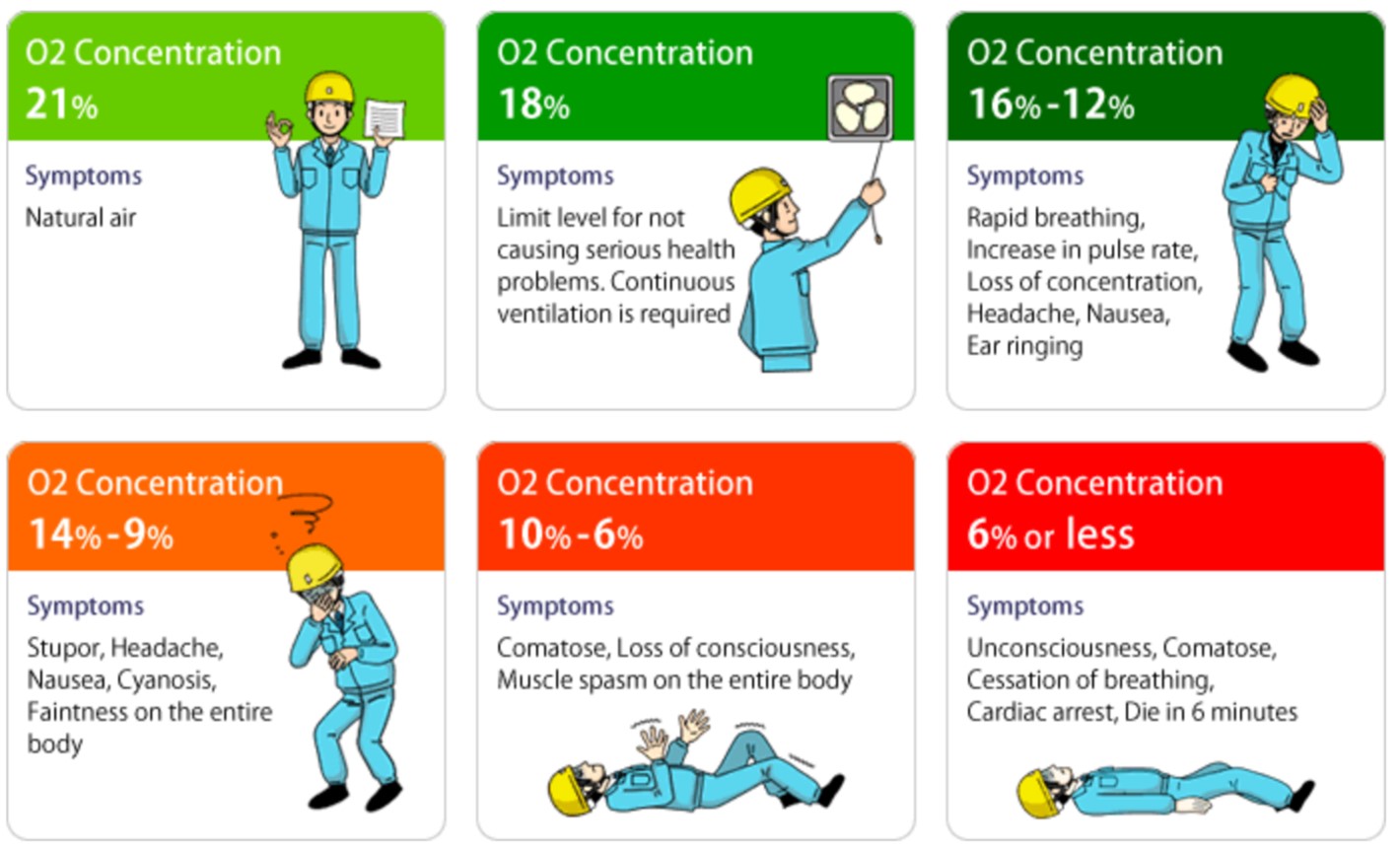
Anoxia is the condition in which the concentration of oxygen in the air is below 18 %.
As defined in Article 2 of the Ordinance on Prevention of Anoxia, etc. (in Japan)
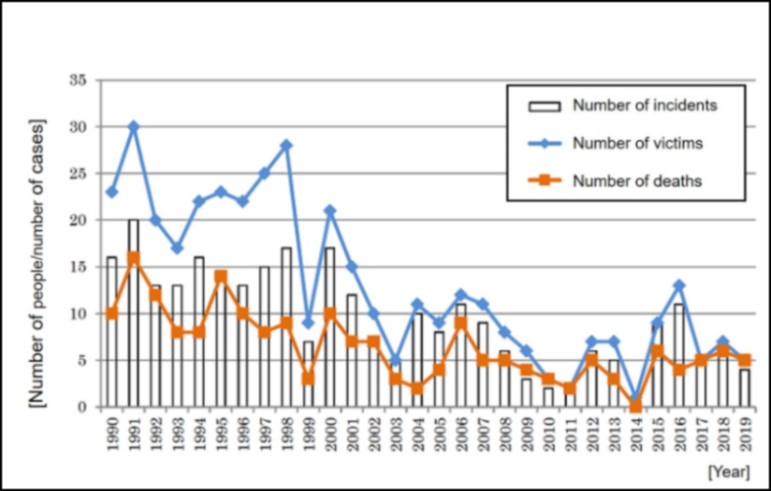
Data on industrial accidents involving oxygen deficiency (1990 - 2020) (in Japan)
Extract from Ministry of Health, Labour and Welfare Basic Industrial Safety Notification No. 0707-3
- Main causes of oxygen deficiency -
Oxygen consumption
Oxidation of metals, ore, and subsoil iron, human respiration, respiration by cereals, fruits, vegetables, and timber, oxidation of paints and solvents
Influx of inert gases or coolants
Influx of N2 gas (including liquid nitrogen), argon gas, or CO2, influx or buildup of coolants such as fluorocarbons
Release of combustible gases
Release of methane gas during excavation, gas leaks from fuel gas pipes
Higher oxygen concentrations will increase the size and temperature of flames, lower the ignition temperature of combustible materials, and increase combustion speed. All this results in an increased risk of fire.
For humans, continuous inhalation of high oxygen concentrations can lead to convulsions, loss of consciousness, and, in the worst case, death.
Gases that are harmful to humans in the event of inhalation or exposure are called toxic gases. While these gases are dangerous, a wide range of industries depend on their particular characteristics. Stringent legal standards regulate the manufacture, transportation, use, and management of gases. Here, we’ll present information on the types and characteristics of toxic gases, which may prove useful in the unlikely event of an accident or disaster.
While the definition of toxic gas was revised through revision of the ministerial ordinance, according to the Exemplified standards relating to the Regulation on Safety of General High Pressure Gas (in Japan), the alarm setpoints for gas leak detection and alarm equipment remain unchanged, and must be threshold limit values or lower.
Toxic gas (in Japan)
Acrylonitrile, acrolein, sulfur dioxide, arsine, monosilane, monomethylamine, hydrogen sulfide (33 gases listed)
and other gases specified as toxic substances in Article 2-1 of the Poisonous and Deleterious Substances Control Act
*Exemplified standards relating to the Regulation on Safety of General High Pressure Gas
Toxic gas (in Japan)(Comparison before and after the 2013 revision)
Previously: 33 listed gases
+ maximum allowable concentration (= threshold limit value) not exceeding 200 ppm
After revision: 33 listed gases
+ toxic substances stipulated in Article 2-1 of Poisonous and Deleterious Substances Control Act
* The only semiconductor material gas classified as a toxic substance is HF.
Threshold limit value (TLV) In accordance with ACGIH (American Conference of Government Industrial Hygienists) recommended values
Workplace air concentrations below which it has been determined most workers suffer no ill effects
・TWA (time weighted average: threshold limit value for workers normally working eight hours a day, 40 hours per week)
・STEL (short-term exposure limit: value that the 15-minute mean cannot exceed)
・C (ceiling value: upper value that cannot be exceeded)
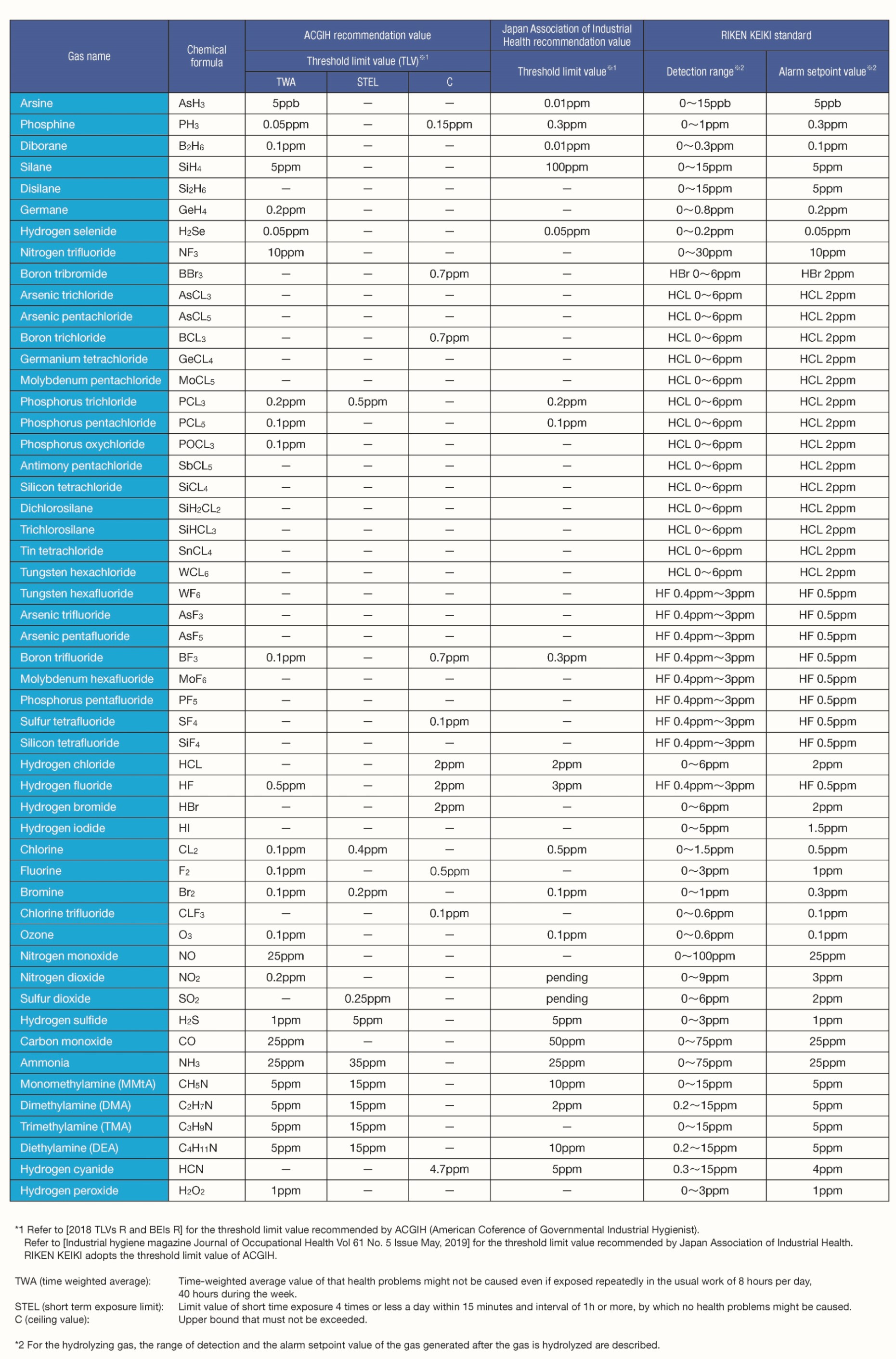
Toxic gas list
This combines with the hemoglobin in red blood cells, impeding the ability to supply oxygen throughout the body. Exposure results in symptoms of toxicity. These symptoms include headache, nausea, dizziness, tinnitus, perspiration, and general lethargy.
CO chemical properties
Colorless, odorless gas
Slightly soluble in water
Molecular weight: 28
Specific gravity: 0.97, virtually identical to air
TLV: 25 ppm (ACGIH: American Conference of Government Industrial Hygienists)
Lower explosive limit (LEL): 12.5 vol%
CO concentration, exposure duration, and symptoms
| CO concentration | Exposure duration and symptoms |
|---|---|
| 25 ppm (0.0025%) |
ACGIH TLV-TWA |
| 0.02 % | Mild headache within 2 - 3 hours |
| 0.04 % | Frontal headache within 1 - 2 hours, rear headache within 2.5 - 3.5 hours |
| 0.08 % | Headache, dizziness, nausea within 45 minutes, loss of consciousness within 2 hours |
| 0.16 % | Headache, dizziness, nausea within 20 minutes, loss of consciousness within 2 hours |
| 0.32 % | Headache, dizziness within 5 - 10 minutes, death within 30 minutes |
| 0.64 % | Headache, dizziness within 1 - 2 minutes, death within 10 - 15 minutes |
| 1.28 % | Death within 1 - 3 minutes |
From Work Environment Measurement Handbook
This gas irritates the skin and mucous membranes due to its high water solubility and reduces oxygen partial pressure in the lungs by inhibiting the action of cytochrome oxidase, a transmembrane molecule, thereby causing breathing difficulties (cellular level oxygen deficiency). ⇒ This is accelerated at higher concentrations, potentially resulting in collapse/loss of consciousness.
H2S chemical properties
Colorless, transparent gas with rotten egg odor
Highly soluble in water (0.25 g/100 ml at 40 °C)
Molecular weight: 34
Specific gravity: 1.190; heavier than air
TLV: 1 ppm (ACGIH: American Conference of Government Industrial Hygienists)
Lower explosive limit: 4 vol%
- Hydrogen sulfide poisoning -
This refers to the condition in which symptoms are observed due to the inhalation of air containing hydrogen sulfide at concentrations of more than 10 parts per million (= 10 ppm).
* As defined in Article 2 of the Ordinance on Prevention of Anoxia, etc.
Hydrogen sulfide concentration, effects, and toxicity
| Concentration (ppm) | Effects and toxicity |
|---|---|
| 0.025 | Threshold at which odor can be detected. This varies from individual to individual.

|
| 0.3 | Odor clearly detectable |
| 3 - 5 | Unpleasant odor of moderate strength |
| 10 | Lower limit of irritation to eye membranes. Threshold limit value. |
| 20 - 40 | Strong but not unbearable odor. Lower limit of irritation to lung membranes. |
| 100 | Sense of smell dulled after 2 - 15 minutes Irritation of eyes and airways within 1 hour. Death may result after continuous exposure for 8 - 48 hours. |
| 170 - 300 | Threshold for avoiding serious health damage after 1 hour exposure. |
| 400 – 700 | Risk to life after exposure for 30 minutes - 1 hour |
| 800 - 900 | Rapid loss of consciousness, respiratory failure, death |
| 1,000 |
Immediate loss of consciousness, death

|
Gases that burn in air or oxygen are called combustible gases. These gases are used in spray cans and other products, both in industry and in the home. They encompass a wide range of types and properties. Since these substances can cause gas explosions and other hazardous incidents, various regulations govern their use. Here, we’ll discuss the types and characteristics of combustible gases.
The Regulation on Safety of General High Pressure Gas defines combustible gases as follows:
・Gases for which the lower explosive limit (explosive limit when mixed with air; the same hereinafter) is 10 % or less
・Gases for which the difference between the upper and lower explosive limits is 20 % or greater
Lower explosive limit (LEL): Concentration in air above which explosions may occur
Upper explosive limit (UEL):Concentration in air below which explosions may occur
Example: Explosive range of hydrogen
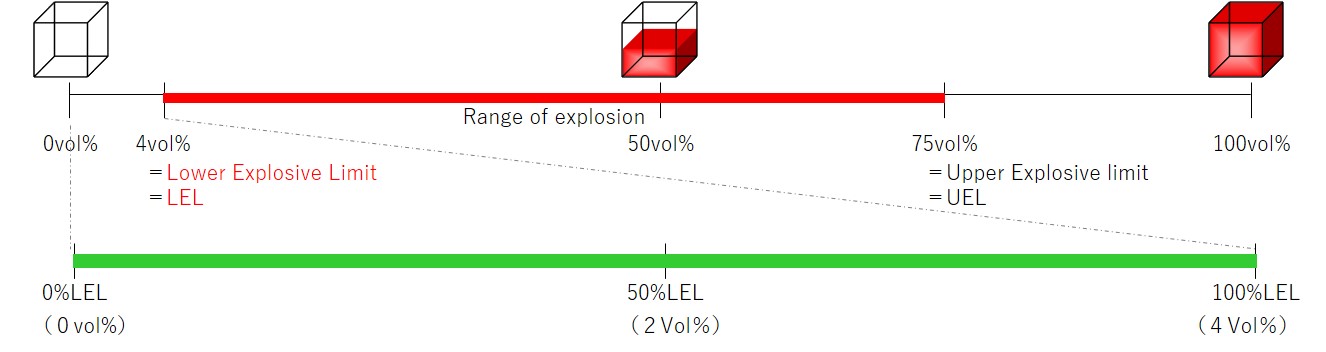
・%LEL is the concentration expressed as a percentage of the lower explosive limit
・The lower explosive limit varies for different gases. (For example, for methane the LEL is 5 vol%; for isobutane, the LEL is 1.8 vol%)
Combustible gas list
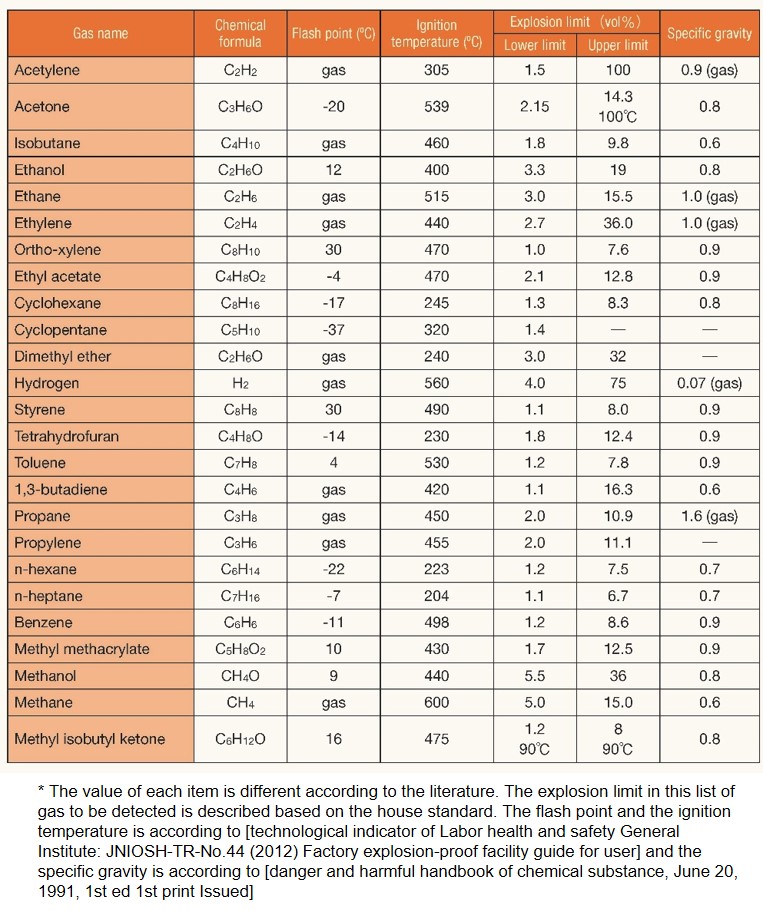
- Methane (CH4) gas properties and characteristics -
・Readily combustible (relatively narrow combustion range)
Combustion range: 5 vol% - 15 vol%
・Will not readily or spontaneously ignite
Ignition point (spontaneous ignition temperature: 537 °C
・Lighter than air
Molecular weight: 16.04; specific gravity (compared to air): 0.6
・The main constituent of natural gas (including shale gas and methane hydrate)
Widely used as fuel gas
・Colorless, odorless, and not harmful to humans
・Has a global warming coefficient approximately 25 times greater than CO2, and is claimed to be a cause of global warming due to
its presence in gases emitted by ruminant animals such as cows, sheep and goats.
Research is underway in numerous countries into ways to reduce emissions to protect the global environment.
Hydrogen (H) is the lightest and most abundant element found on Earth.
Hydrogen is a combustible gas with a low minimum ignition energy and a broad explosive range when mixed with air. Since it does not generate the greenhouse gas CO2 when burned, unlike other typical combustible gases such as methane and propane, it has become the focus of attention in recent years as a potential clean energy source.
Typical combustible gases: CH4 + 2O2 → 2H2O + CO2 (greenhouse gas) emission
Hydrogen gas: H2 + 1/2O2 → H2O
- Hydrogen (H2) gas properties and characteristics -
・Readily combustible (wide combustible range)
Combustible range: 4 vol% - 75 vol%
・Will not readily or spontaneously ignite
Ignition point (spontaneous ignition temperature): 570 °C
・The lightest of all gases on Earth
Molecular weight: 2.017; specific gravity (compared to air): 0.07
・Colorless, odorless, and not harmful to humans
・Does not generate CO2 when burned
Low environmental load
・Plentiful resource; most abundant element on Earth
Manufacturing method: Electrolysis of water (e.g., seawater), extraction from fossil fuels, byproduct gas from steel manufacturing,
etc.
- Topic: Technology enabling a hydrogen society -
While hydrogen is a combustible gas related with risk of explosion, it is seen as potentially playing a major role in preventing global warming, as it does not generate CO2 emissions, unlike other fossil fuels.
- Hydrogen usage examples -
Power to Gas
Technology for manufacturing hydrogen and synthetic methane using power from renewable energy (e.g., solar and wind power). Research and demonstration testing is currently underway around the world to establish an environmentally friendly low-carbon society.
Methanation
Technology for synthesizing methane (CH4), the main constituent of natural gas, from CO2 and H2. CH4 synthesized using CO2 as a raw material can be considered a carbon-neutral fuel. In contrast to H2 directly, synthesized CH4 would allow use of the existing natural gas infrastructure.
FCVs (fuel cell vehicles)
Vehicles that run on electricity generated by a chemical reaction between hydrogen and oxygen in a fuel cell to drive a motor It is anticipated as an environmentally friendly zero-emission technology, since it generates no CO2 is emitted, and all that is with water its only emission.
Organic hydride technology
Technology facilitating storage and transportation using MCH (methylcyclohexane), with a volume approximately 1/500th of hydrogen, produced through a catalytic reaction between hydrogen and toluene This will allow storage of hydrogen for extended periods and safe, simple hydrogen transport in greater volumes.
Other hazardous gases are also used in various industrial and domestic settings. Here, we’ll explain the terms specific gravity of vapor and vapor pressure, key factors in detecting leaks of CO2 gas - something highlighted as one of the SDGs.
Carbon dioxide has low toxicity and rarely causes respiratory symptoms. In high concentrations, it produces anesthetic effects and can cause death by suffocation.
CO 2 chemical properties
Colorless, odorless gas
Non-combustible
Soluble in water Solubility: 90.1 mL/100 mL, 20 °C
Molecular weight: 28 Specific gravity: 1.5
TLV: 5,000 ppm (ACGIH: American Conference of Government Industrial Hygienists)
Not defined as a toxic gas in the Regulation on Safety of General High Pressure Gas
Act on Maintenance of Sanitation in Buildings
(abbreviation: Building Maintenance Act)
(in Japan)
The owners, occupants, and managers of specified buildings exceeding a specific total area (e.g., department stores, offices, schools, hotels) must maintain buildings in accordance with standards stipulated by government ordinances (Article 4)
Building Environmental Hygiene Management Standards (Government Ordinance Article 2)
Air conditioning equipment is required to supply air purified so that carbon dioxide concentrations do not exceed 1,000 ppm.
CO2 concentration and symptoms
| CO2 concentration | symptoms |
|---|---|
| 0.1 % (1,000 ppm) | Building hygiene management standard |
| 0.5 % | TLV-TWA threshold at which harmful effects to the body are caused by 8 hours exposure daily |
| 1 - 2 % | Causes discomfort |
| 3 - 4 % | Irritation of the respiratory center resulting in symptoms such as increased respiratory rate, increased pulse and blood pressure, headache, and dizziness |
| 6 % | Respiratory difficulties |
| 7 - 10 % | Loss of consciousness within a few minutes, cyanosis, and death |
Aging of CO2 gas concentration
The following graph shows changes in concentrations of CO2 gas in the atmosphere in Japan between 1987 and 2020.
The concentration of CO2 gas is increasing annually, with levels of 410 ppm or more of CO2 in the atmosphere recorded in recent years.
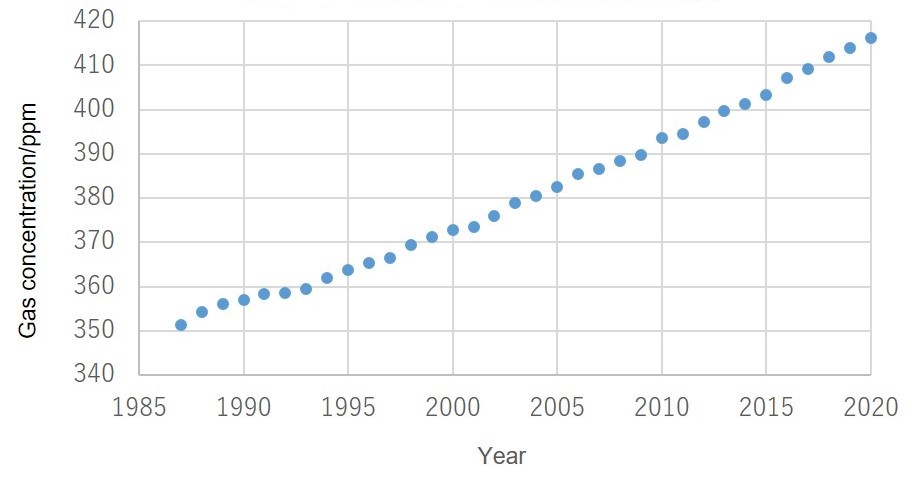
Based on Meteorological Agency measurement data from Ryori, Ofunato, in Iwate Prefecture
Carbon dioxide (CO2) is known as one of the greenhouse gases that cause global warming. Signatory nations of the 2016 Paris Agreement* are pushing ahead with decarbonization measures to reduce CO2 emissions.
* International initiative to tackle climate change issues from 2020 onward at the Conference of the Parties to the United Nations Framework Convention on Climate Change (known as “COP”) held in Paris in 2015.
Details on ventilation methods for improving poorly ventilated enclosed spaces (leaflet) published on the Ministry of Health, Labour and Welfare website (revised April 3, 2020) indicate that promoting indoor ventilation in accordance with the Building Maintenance Act standard (not more than 1,000 ppm carbon dioxide) constitutes one measure for preventing the risk of infection clusters.
This indicates the ratio of the density of a particular gas with respect to the density of air under standard conditions. This is one of the factors that must be taken into consideration when considering where to install gas detectors.
For gases heavier than air (specific gravity of vapor > 1)
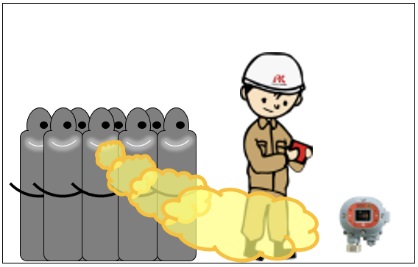
If gas leaks occur, gas is likely to accumulate at lower points
⇒ Gas detectors should be installed at lower points.
For gases lighter than air (specific gravity of vapor < 1)
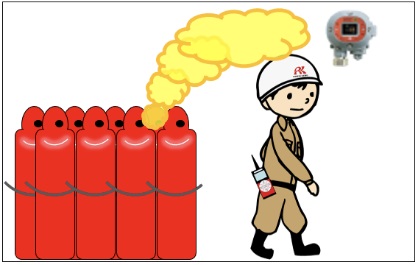
If gas leaks occur, gas is likely to accumulate at higher points
⇒ Gas detectors should be installed at higher points.
This refers to the pressure at which a liquid changes into a gas at a specific temperature. If vapor pressure data is available from documents such as SDS (safety data sheets) for VOCs or other organic solvents, this enables calculation to determine the concentration at which the substance will vaporize (at a given temperature).
Example calculation:
Ethyl alcohol Vapor pressure: 5.8 kPa/20 °C
If atmospheric pressure is 101.3 kPa
Vapor pressure concentration (%) = Vapor pressure ÷ Atmospheric pressure = 5.8 ÷ 101.3 ≒ 5.73 vol%
Thus, the vapor pressure concentration of ethyl alcohol at 20 °C is approximately 5.73 vol%